Table of Contents
Sleep apnea is a pervasive condition that affects millions of people, impacting their quality of life and overall health. Among various treatment options available, an innovative FDA-approved solution has emerged to target obstructive sleep apnea (OSA): Inspire Sleep Apnea Treatment.
OSA is characterized by pauses in breathing or shallow breaths during sleep, and traditional treatment methods often involve the use of continuous positive airway pressure (CPAP) devices.
However, not everyone finds success with CPAP machines, experiencing discomfort or challenges with consistent usage. This is where Inspire Sleep Apnea Treatment enters the picture, offering an alternative solution.
Inspire’s implantable upper airway stimulation (UAS) device works by monitoring the patient’s breathing and sending a gentle electrical pulse to the nerve that controls tongue motor function.
The stimulation moves the tongue forward, clearing the airway for patients to breathe properly while asleep. Sleep Foundation highlights its potential for those seeking an effective and comfortable solution to OSA.
Overview of Inspire Sleep Apnea Treatment
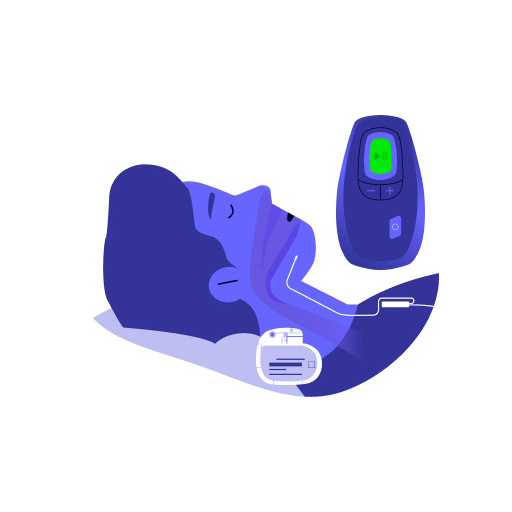
Inspire Sleep Apnea Treatment is an innovative approach to managing moderate-to-severe obstructive sleep apnea. It uses upper airway stimulation technology to help patients with sleep apnea breathe better and sleep more comfortably at night.
Upper Airway Stimulation
The Inspire Sleep Apnea Treatment uses a small, surgically implanted device to stimulate the hypoglossal nerve, which in turn causes the throat and other muscles to open up the airway.
This allows patients to breathe more easily and sleep better. The device is controlled by the patient with a remote, making it convenient and easy to use.
FDA-Approved
Inspire Sleep Apnea Treatment was first approved by the FDA in 2014, meaning that it has undergone rigorous testing to ensure both its safety and effectiveness for treating sleep apnea.
Innovation
Unlike traditional treatments for sleep apnea, such as Continuous Positive Airway Pressure (CPAP) machines, Inspire Sleep Apnea Treatment does not require the use of masks or hoses. This makes it a more comfortable and less intrusive option for those who struggle with CPAP therapy.
The treatment has been shown in studies to significantly reduce the number of sleep apnea events experienced by patients, improving their overall sleep quality and health.
Candidates for Inspire
The Inspire Sleep Apnea Treatment is not suitable for every individual with sleep apnea. There are specific criteria that a person must meet in order to be considered a good candidate for this treatment.
Apnea-Hypopnea Index (AHI)
An important factor in determining eligibility for Inspire is the individual’s Apnea-Hypopnea Index (AHI) score. The AHI is a measurement of the severity of sleep apnea, with the number of apnea and hypopnea events per hour of sleep being recorded.
To be eligible for Inspire, a person must have an AHI score between 15 and 65, signifying moderate to severe sleep apnea
Body Mass Index (BMI)
Another crucial aspect to consider before opting for Inspire is the individual’s Body Mass Index (BMI). Although specific BMI requirements may vary depending on the healthcare provider, having a lower BMI is usually preferable. A reduced BMI may allow for better treatment outcomes and a higher likelihood of treatment success.
Weight Loss
Weight loss can be an essential factor in improving sleep apnea symptoms and overall sleep health. For some individuals, losing weight may be a necessary step before becoming eligible for Inspire treatment. Achieving a healthy weight can help improve sleep apnea symptoms and may enhance the efficacy of the Inspire device.
Comfortable Sleep
Candidates for Inspire should also consider their comfort during sleep. The device is implanted under the skin and may take some getting used to. Additionally, users will need to learn how to operate the remote control that activates the device.
Ensuring a comfortable sleep and adjusting to the changes brought on by the Inspire device is necessary for a successful treatment experience.
The Implantation Procedure
The Inspire sleep apnea treatment involves a surgical procedure to implant the device, which consists of a generator, wire, and stimulation lead. This section provides an overview of the different steps involved in the implantation process.
Surgical Procedure
The surgery for implanting the Inspire sleep apnea device is an outpatient procedure performed under general anesthesia. The surgeon makes several small incisions on the side of the chest and neck to access the necessary insertion points. The entire procedure typically takes around two to three hours.
Generator and Wire Placement
The generator, which acts as the main control unit for the device, is placed under the skin on the chest’s upper right side. It is responsible for sending mild electrical signals to the stimulation lead. A wire is then inserted and tunneled under the skin, connecting the generator to the stimulation lead placed in the neck.
Stimulation Lead
The stimulation lead is implanted near the hypoglossal nerve, which controls the movement of the tongue. By sending electrical signals from the generator, the lead stimulates the nerve to keep the airway open during sleep, thus reducing the occurrence of sleep apnea events. It is carefully positioned to ensure proper activation of the tongue muscle while minimizing discomfort.
Monitoring and Adjustments
Inspire Sleep Apnea Therapy involves continuous monitoring and adjustments to ensure the most effective treatment for each individual. This section discusses remote control usage, the therapy’s effectiveness, and airflow assessment during the treatment process.
Remote Control Usage
Patients using Inspire Sleep Apnea Therapy are equipped with a remote control that allows them to manage their device settings. The remote enables users to turn the device on and off, adjust the stimulation level, and pause the system during the night if required.
This level of control empowers patients to actively participate in their treatment journey and make adjustments based on their comfort and needs.
Effectiveness
The effectiveness of Inspire Sleep Apnea Therapy depends on the accurate assessment and fine-tuning of device settings. Over time and through regular follow-ups with the sleep specialist, adjustments may be required to achieve optimal therapy results.
It was reported that one patient’s events were reduced to seven an hour, but further adjustments were made with the goal of achieving even better results.
It’s essential to collaborate closely with the sleep specialist as they guide the process to find the most beneficial settings. In some cases, it may take several months of adjusting different settings to drastically reduce sleep apnea events.
Airflow Assessment
An essential aspect of Inspire Sleep Apnea Therapy is regular airflow assessment. The device continuously monitors the patient’s breathing patterns throughout the night and adjusts stimulation as needed.
This responsiveness results in more effective treatment by adapting to the user’s unique breathing patterns and ensuring proper airflow throughout the entire sleep cycle.
Overall, patients using Inspire Sleep Apnea Therapy benefit from a highly personalized and adaptive system that enables them to have control over their treatment while constantly adjusting to provide the best possible results.
Risks and Recovery
Discomfort and Swallowing Difficulty
Following the Inspire sleep apnea therapy, patients might experience some temporary discomfort around the implant site, as well as difficulty swallowing or speaking. It was reported that these symptoms tend to be temporary, but it is essential to monitor and discuss them with a healthcare provider if they persist or worsen.
Post-Operative Pain Medication
Post-operative pain medications are often prescribed to manage any discomfort related to the procedure. These medications can be tailored to individual patients based on their level of pain and potential risks.
It is crucial to follow the healthcare provider’s instructions to ensure proper pain management and prevent unwanted side-effects, such as dependence or excessively suppressing the central nervous system.
Recovery from the Inspire sleep apnea therapy implantation may vary from one patient to another due to individual differences in healing and tolerating the device. If patients experience persistent throat discomfort or difficulty swallowing, it is essential to consult their healthcare provider for appropriate evaluation, guidance, and intervention if necessary.
Potential Limitations and Future Developments
While Inspire Sleep Apnea Treatment has shown promising results in treating obstructive sleep apnea (OSA), it may not be suitable for all cases or types of sleep apnea.
Central Sleep Apnea
It is important to note that Inspire is primarily designed for OSA, which occurs when the throat muscles relax, blocking the airway during sleep. However, another sleep apnea type known as central sleep apnea (CSA) is a result of the brain not sending proper signals to the muscles controlling breathing.
As Inspire focuses on stimulating throat muscles, it may not be an effective treatment for CSA cases.
A suitable option for patients with CSA would need to address the neurological aspects of the condition. More research and development of treatments specifically tailored to CSA may be necessary for the future.
Further Research and Improvements
Inspire has shown significant potential in reducing sleep apnea events for many people suffering from OSA. According to a study, 66% of subjects achieved a reduction of at least 50% in their apnea-hypopnea index (AHI) alongside improvements in daytime sleepiness and functional outcomes.
Despite the positive results, there is still room for further advancement in the treatment. Patients and medical professionals should be aware of potential adverse events associated with the Inspire implant, which may include device malfunctions or iatrogenic injuries.
Ongoing research and development will be crucial in addressing these issues and improving the safety and efficacy of the treatment.
Moreover, expanding research to explore UAS therapy for different demographics, including age or BMI subsets, may provide a clearer understanding of the treatment’s suitability for other populations.
As Inspire continues to evolve and address its limitations, its role in sleep apnea management may solidify further, providing a valuable alternative to traditional treatments such as CPAP therapy.
Clinical Studies and Success Rates
Inspire Sleep Apnea treatment has been extensively studied in clinical trials to assess its effectiveness and safety. One of the most significant factors in evaluating the success of this therapy is the reduction in the Apnea-Hypopnea Index (AHI).
Study Outcomes
Research conducted on patients with severe obstructive sleep apnea who underwent Inspire therapy demonstrated a statistically significant improvement in their AHI scores. The median AHI for 20 participants after implantation decreased by 37.7, with a median total AHI of 2.1 events per hour.
Another study found that Inspire obtained a surgical success rate of 72.4%, with 75% success at 60-month follow-up. Additionally, patient adherence to the treatment in Inspire implantable Hypoglossal nerve stimulation (HNS) therapy was found to be as high as 86% after 12 months.
Effectiveness
The Inspire Sleep Apnea treatment involves implantation of a device in the upper chest, which stimulates the hypoglossal nerve to maintain an open airway during sleep. This therapy is shown to be particularly effective in reducing the symptoms of sleep apnea, as demonstrated by the study outcomes mentioned above.
Treatment planning includes a drug-induced sleep endoscopy, which allows doctors to observe patients under sedation and better understand sleep apnea severity and suitability for Inspire therapy.
Overall, the clinical studies and data gathered on Inspire Sleep Apnea treatment demonstrate its potential effectiveness in improving sleep apnea symptoms and reducing AHI scores for suitable patients.
Customer Reviews
Inspire Sleep Apnea Therapy has received mixed reviews from patients who have tried the treatment. Some users have found success with the therapy, reporting significant improvement in their sleep quality and daytime functioning. Consumer Health Digest rates it 1.7 out of 5 based on 24 reviews, illustrating the varied experiences of patients who underwent Inspire Sleep Therapy.
On the positive side, many patients highlight the benefits of not having to wear a CPAP mask during sleep. Some also mention an increased overall wellbeing, reduced daytime sleepiness, and improved concentration after using the Inspire device. Additionally, the therapy’s minimally invasive nature and the ability to adjust its settings remotely contribute to its appeal for some users.
However, there are also negative reviews and experiences shared by patients. Common concerns include the high cost of the treatment, potential complications related to the surgical implant, and the need for periodic adjustments. Some users also report experiencing discomfort or side effects, such as mild pain or changes in their voice.
In conclusion, the Inspire Sleep Apnea Therapy offers a promising alternative to CPAP for some patients with obstructive sleep apnea. However, as the mixed customer reviews demonstrate, the therapy may not be suitable for everyone.
It is essential to consult with a qualified healthcare professional to discuss available treatment options and determine if Inspire Sleep Therapy might be appropriate for you.
Insurance and Costs
Medicare and Private Insurance Companies
Inspire sleep apnea treatment is covered by Medicare and most private insurance companies, provided that the criteria for the surgery are met. More than 140 private insurance firms now offer coverage for the implant, making it more accessible to a wider range of patients.
Resources
Each insurance provider has its own specific requirements when it comes to covering the Inspire Sleep apnea implant. Patients are advised to consult with their respective insurance companies to determine their eligibility for coverage.
A helpful resource for understanding the cost and eligibility process for Inspire Sleep can be found on the official Inspire Sleep website. This webpage provides an overview of the criteria and requirements that may be necessary for coverage.
Deductibles
For those whose insurance plans do provide coverage for Inspire Sleep apnea treatment, there may still be out-of-pocket expenses associated with deductibles. These costs can range between $1,000 to $2,000.
It is important to note that the total cost for the Inspire sleep apnea implant is estimated to be around $20,000, not including the operation itself. Patients should factor in these expenses when considering whether the treatment is the right choice for them.
Comparison to Alternatives
Continuous Positive Airway Pressure (CPAP)
The most common and widely prescribed treatment for obstructive sleep apnea (OSA) is Continuous Positive Airway Pressure (CPAP). A CPAP machine delivers a continuous flow of air pressure through a mask, keeping the airways open and preventing apneas during sleep.
While CPAP is highly effective in treating OSA, some patients find it uncomfortable or difficult to tolerate, leading to low adherence rates.
In comparison, Inspire Sleep Apnea Treatment is an implantable device targeting patients who struggle with CPAP adherence. Inspire works inside the body, stimulating key airway muscles to keep the airway open.
It is a more invasive treatment option than CPAP, but may provide a viable alternative for patients who cannot tolerate traditional CPAP therapy.
Oral Appliances
Oral appliances are another treatment option for patients with sleep apnea. These custom-fitted devices, usually provided by dentists, work by repositioning the lower jaw, tongue, or soft palate to maintain an open airway during sleep.
Oral appliances can be effective in treating mild to moderate OSA, but may not be suitable for all patients or for those with severe sleep apnea. They can also cause temporary side effects, such as jaw pain or dental issues.
While Inspire does require surgery for implantation, it addresses the root cause of OSA by directly stimulating airway muscles. This may be a more effective solution for those with more severe forms of sleep apnea, as well as patients who have not seen success with oral appliances.
Positional Therapy
Positional therapy involves the patient sleeping in specific positions to reduce the likelihood of airway obstruction. For some patients, simply sleeping on their side or using special pillows to maintain proper head and neck alignment can help alleviate OSA symptoms.
However, not all patients find relief from positional therapy, and it may be difficult to maintain these sleep positions throughout the night.
Inspire Sleep Apnea Treatment addresses OSA through a different mechanism, by actively stimulating the airway muscles to maintain an open airway even as the patient changes sleep positions. For those who have not found success with positional therapy, Inspire may offer a more consistent and reliable treatment option.
Is It Worth It?
Inspire Sleep Apnea Treatment is an FDA-approved implantable upper airway stimulation (UAS) device designed for individuals suffering from obstructive sleep apnea (OSA).However, the question remains: is it worth the investment?
One of the primary benefits of Inspire is its effectiveness in reducing sleep apnea events. In an Inspire-funded study, two-thirds of the participants experienced a reduction of at least 50% in sleep apnea events within 12 months of receiving the implant.
Unlike most other sleep apnea therapies, Inspire is an implant that requires invasive surgical intervention. While some low-level discomfort may be associated with the procedure, there is a slight risk of infection, as with any invasive method.
That being said, Inspire is considered low maintenance and does not interfere with the user’s daily routine, promoting better sleep and breathing.
However, Inspire Sleep Apnea Treatment may not be suitable for everyone. Not all individuals with sleep apnea qualify for this type of treatment, and the financial aspect can be substantial depending on your health insurance coverage.
Given the benefits and considerations, the decision to undergo Inspire Sleep Apnea Treatment may largely depend on individual circumstances, including the severity of sleep apnea, other treatment options, and personal preferences.
Consultation with a qualified medical professional is recommended to determine if Inspire is the right choice for your specific situation.
Conclusion
Inspire Sleep Apnea Treatment is an innovative approach to managing moderate to severe obstructive sleep apnea. This implantable device works through nerve stimulation, moving the tongue forward, and ensuring proper airflow during sleep.
The treatment has been shown to be effective in reducing sleep apnea events, as research has found an average reduction of 68% in the Apnea-Hypopnea Index (AHI) over 12 months.
However, it is important to note that Inspire is generally considered a secondary treatment, recommended for those who struggle with CPAP adherence or cannot tolerate other first-line treatments. Despite its benefits, the device still requires surgery for implantation, and not all patients may be eligible for the procedure.
Before considering Inspire, it is essential to consult with a sleep specialist to determine whether this treatment is appropriate for each specific case. As with any medical treatment, individual results may vary, and long-term success will depend on proper adjustments, follow-up care, and patient commitment to managing their sleep apnea.
FAQ
How does Inspire Sleep Apnea Treatment work?
Inspire is an upper airway stimulation device that is implanted surgically and controlled by the patient with a remote. It stimulates the hypoglossal nerve, which in turn causes the throat and other relevant muscles to open up the airway again during sleep.
It also monitors the patient’s breathing pattern throughout the night and aids in adjusting the stimulation to promote better sleep and breathing.
How effective is Inspire for treating sleep apnea?
In one Inspire-funded study, two-thirds of the participants who got the implant experienced sleep apnea events reduced by at least 50 percent at 12 months.
What makes Inspire different from traditional CPAP devices?
Unlike CPAP devices that use pressurized air to clear the airway, Inspire uses nerve stimulation. The surgically implanted device monitors the patient’s breathing and sends a gentle pulse to the nerve that controls tongue motor function, moving it forward and out of the way so breathing can occur properly.
What should patients expect during an appointment?
When preparing for an appointment, patients should bring any relevant medical documents with them. It is important to discuss with the medical professional any personal medical history, sleep issues, and potential candidacy for the Inspire treatment.
References
- Bestourous, Daniel E., et al. “Adverse Events Associated with the Inspire Implantable Hypoglossal Nerve Stimulator: A MAUDE Database Review.” American Journal of Otolaryngology, vol. 41, no. 6, Nov. 2020, p. 102616, https://doi.org/10.1016/j.amjoto.2020.102616. Accessed 29 Apr. 2023.
- Boyd, Scott B., et al. “Effective Apnea-Hypopnea Index (“Effective AHI”): A New Measure of Effectiveness for Positive Airway Pressure Therapy.” Sleep, vol. 39, no. 11, 1 Nov. 2016, pp. 1961–1972, pubmed.ncbi.nlm.nih.gov/27568799/, https://doi.org/10.5665/sleep.6224.
- Costantino, Andrea, et al. “Hypoglossal Nerve Stimulation Long-Term Clinical Outcomes: A Systematic Review and Meta-Analysis.” Sleep and Breathing, vol. 24, no. 2, 15 Aug. 2019, pp. 399–411, https://doi.org/10.1007/s11325-019-01923-2. Accessed 24 Jan. 2023.
- “FDA Approves Inspire Upper Airway Stimulation Therapy for Sleep Apnea.” American Academy of Sleep Medicine – Association for Sleep Clinicians and Researchers, 1 May 2014, aasm.org/. Accessed 29 Apr. 2023.
- Heiser, Clemens, et al. “Post-Approval Upper Airway Stimulation Predictors of Treatment Effectiveness in the ADHERE Registry.” The European Respiratory Journal, vol. 53, no. 1, 3 Jan. 2019, pp. 1801405–1801405, www.ncbi.nlm.nih.gov/pmc/articles/PMC6319796/, https://doi.org/10.1183/13993003.01405-2018. Accessed 28 Apr. 2023.
- Inspire Sleep. “Positive Patient Outcomes Data on Inspire Therapy Highlighted at SLEEP 2016.” 2016. https://www.inspiresleep.com/.
- Pinto, Venessa L., and Sandeep Sharma. “Continuous Positive Airway Pressure.” PubMed, StatPearls Publishing, 2021, pubmed.ncbi.nlm.nih.gov/29489216/. Accessed 29 Apr. 2023.
- Rana, Abdul M., and Abdulghani Sankari. “Central Sleep Apnea.” PubMed, StatPearls Publishing, 2023, pubmed.ncbi.nlm.nih.gov. Accessed 29 Apr. 2023.
- Romero-Corral, Abel, et al. “Interactions between Obesity and Obstructive Sleep Apnea.” Chest, vol. 137, no. 3, Mar. 2010, pp. 711–719, www.ncbi.nlm.nih.gov/pmc/articles/PMC3021364/, https://doi.org/10.1378/chest.09-0360. Accessed 29 Apr. 2023.
- Slowik, Jennifer M., and Jacob F. Collen. “Obstructive Sleep Apnea.” PubMed, StatPearls Publishing, 2020, www.ncbi.nlm.nih.gov/books/NBK459252/. Accessed 29 Apr. 2023.
- Strollo, Patrick J., et al. “Upper Airway Stimulation for Obstructive Sleep Apnea: Durability of the Treatment Effect at 18 Months.” Sleep, vol. 38, no. 10, 1 Oct. 2015, pp. 1593–1598, www.ncbi.nlm.nih.gov/pmc/articles/PMC4576333/, https://doi.org/10.5665/sleep.5054.
- “What Is Inspire Sleep Apnea Treatment and Does It Work?” Sleep Foundation, 7 Apr. 2022, www.sleepfoundation.org/sleep-apnea.
Next, check out some recent reviews you might find useful:

Leave a Reply